|
CLEANING SOLUTIONS
By James Moss (Littleton, Massachusetts) c. 1998 (All rights reserved by the author.) (re-created, updated and used with permission of the author) (originally published: Horological Times, February 1998)
About the Author: James Moss is a Professional Associate of the American Institute for the Conservation of Historic and Artistic Works (AIC). He has been in full-time private practice as a horological restorer since 1969 and as a Professional Associate Conservator since 1993. He is a Director of New England Conservators Association. He is a member of the American Watchmakers-Clockmakers Institute (AWI), NAWCC, British Horological Institute (BHI), and the United Kingdom Institute for Conservation of Historic and Artistic Works (UKIC). He was a co-founder of NAWCC Chapter 87. He lectured for many years on the technical aspects of clock repair and restoration for NAWCC Chapters 8 and 87. He has designed and built over thirty sundials of varying designs. He holds an ASEE from Wentworth Institute of Technology and served as a consultant to the Arthur D. Little Corp. on micro-mechanical mechanisms for the U.S. Navy.
ARE AMMONIATED CLEANING SOLUTIONS SAFE FOR CLOCKS AND WATCHES?
Abstract: Ammoniated solutions for cleaning watches and clocks have been promoted and used for at least the last 90 years with full knowledge that ammonia can cause stress corrosion cracking of hardened brass. It is time to consider the effects that ammoniated solutions could be having on the longevity of our horological objects and to re-think the philosophies of the cleaning process.
Using any cleaning solutions that contain ammonia or any of its related compounds such as amines1 or nitrogen bearing chemicals, even in small amounts, to clean or polish brass components of clocks and watches could damage the very objects you are seeking to preserve.
Ammonia (and its related compounds or derivatives) is a known producer of STRESS CORROSION CRACKING (SCC) of hardened brass objects such as clock pawl springs and gears. There is so much scientific evidence available regarding the effect that ammonia can have on hardened brass that this is no longer a debatable point – it is a fact.
SCC can damage hardened brass when the brass is exposed to ammonia, either in a liquid or gaseous form and in very small amounts. Immersion in large quantities of ammonia or in concentrated amounts is not necessary to produce SCC; common household ammonia can produce SCC quite easily! Ammonia will begin to do its damage as soon as the component has been immersed in the cleaning solution; even rinsing under strong sprays will not remove the effects that the ammonia has had upon the brass metal structure. Repeated exposure to ammonia is cumulative and it is especially so if cracking has already begun.
Early brass is not homogenous; it can contain porous sections, bubbles, fissures, slag, and other pollutants that could provide a harbor within the metal for the ammoniated solutions to reside thus increasing the chance for long-term damage. In addition, brass is not a simple combination of copper and zinc. There are many other components that make up “brass” because there is no such thing (outside of a laboratory) as 100% pure copper or pure zinc. Remember that brass has been around since Roman and Greek times and many re-melts have occurred since then. (The brass that you buy directly from the mill today can contain many uninvited ingredients including mercury!) With each re-melt, the chance of introducing contaminates increases. An increase in contaminates will complicate the reactions to any chemicals that are applied to the brass or to which the brass is exposed.
Before the first World War, it was well known (and documented) that ammonia would cause hardened brass objects to develop SCC. During this time, even with this awareness, ammoniated clock cleaning solutions such as Daniels solution (Oleic acid, acetone, and ammonia) have been offered, disseminated, and recommended by many of the most respected names, text books, journals, and institutions in the horological trade (as examples, see recent AWI Horological Times and NAWCC Bulletin articles, J. E. Coleman’s writings, DeCarle writings, and Penman’s book The Clock Repairer’s Handbook, 1992, pg. 35. SCC mentioned but Daniel’s solution recommended). Chances are very high that the clock or watch that you worked on recently was washed with an ammoniated solution at least once since it was made! Many will say that they have never seen SCC, others will say that it only happens to old clocks but not new ones or only on 17th century clocks or only on tall clocks (see addendum at the end of this article for further information about this point) or……….…(and the list is exhaustive), but the fact remains that ammonia causes SCC on hardened brass components regardless of their application (be it a brand name brass gun cartridge or a clock part). Ammonia does not choose a specific year or a type of clock; it doesn’t care what it is as long as it is brass and hardened!
The obvious solution is to keep the hardened components away from ammoniated solutions, but this is easier said than done. How can you tell if a component is hardened? Generally you cannot tell by looking. Hammer or rolling mill marks may be a clue but usually the material is hardened prior to final shaping and finishing. The only positive way to determine “hardness” is with a hardness tester (which can be destructive to small parts). Hardness testing can be expensive and unfeasible if each component of a mechanism is to be tested and hardness testing can, itself, introduce additional stresses to the component.
Some will argue that clock parts are all cast and thus are “soft” and are immune to the effects of ammonia. This is simply not true. Many parts are cast, many parts are hammered, many parts are stamped, many parts are rolled, but the question is, “Which of those parts are the vulnerable ones?” Actually the real question is, “Why take a chance?” The convenience, the low cost, the visual results, and the ease of use that ammoniated solutions provide is simply not worth the risk either in the short or the long term. No clock, historically significant or not, should be exposed to ammoniated cleaning solutions.
Ammoniated solutions are popular because they clean so well. The parts are so bright and shiny that their appearance makes the watch or clock repairer as well as the client feels that the clock has really been cleaned! The parts are bright because the ammonia is etching the surface of the brass parts (read “the ammonia is dissolving the parts right before your eyes”). Some people will argue that the amount of metal removed as a result of this etching is so small that it really doesn’t matter but remember that most of us are amazed that these objects have lasted 100, or 200 or even 300 years. If some metal is removed chemically each time the clock or watch is cleaned, over a long period of time a measurable amount of metal will be removed and over a very long period of time the artifact will disappear! I believe that most of us would like to think that the clock or watch that we are working on will continue to exist long after we have passed away. We could ask ourselves the following question, “What could we be doing to better insure that this object that we are working on will exist 2000 years from now?”
By using the ammoniated solutions, you are compromising the ability to preserve valued antiques and national treasures (many of you work on museum objects or you work or you volunteer at a museum either privately or in conjunction with a horological organization such as the AWI or the NAWCC). Even if you do private work (some feel that doing private work exonerates one from addressing preservation issues), you are still compromising the integrity of the clock or watch because of the effects SCC has on the metallurgical integrity of the mechanical components that make up the clock or watch.
Museums do not house and care for the only historically significant clocks and watches that exist! There are many historically significant clocks and watches in private collections; and there are many ordinary clocks and watches in private collections that will become historically significant in the years to come. It is imperative that the horological community takes steps now to reduce the effects that the use of ammoniated solutions could have (read “already may have had”) on the integrity of these clocks and their components.
In a general sense, without getting into the specifics of “how” (because the “hows” are still being discussed by metallurgists and scientists even today), SCC will cause intra- or trans-crystalline cracking of the part and ultimate mechanical failure (the cracking can be seen with a microscope at 60X power). You may have seen failures of brass click springs, canoe springs, wheel crossings or teeth and brass plates (the failure looks like the part just broke) and wondered why this had happened. Most likely exposure to ammonia was the cause.
At this point in time, there are no safe cleaning solutions commercially available. Even the solutions that state that they are non-ammoniated can have an “amine” component in the mixture.
Is there a solution to this dilemma?
Yes, there is, but some sacrifices will need to be made (such as no more bright shiny parts and possibly the application of more elbow grease).
After sounding a warning like this, one would expect, at least in the present day, that corrective solutions to the problem would be forthcoming or provided immediately within the framework of the article itself and the problem would be solved. Unfortunately that is not the case here. The best that I can offer at this time is a cleaning solution that is, as far as I have been able to determine, safe to use on hardened brass components as well as on hardened or soft steel or iron. I must caution you that at this time – this solution has not been tested in a metallurgical laboratory and thus I strongly urge the use of caution when using it until a complete analysis of its reaction with the metals can be done. Sources for the chemicals are listed at the end of this article.
|
My Solution This is my solution – I am choosing to err on the CONSERVATIVE side and to eliminate all solutions that contain ammonia, amines, or their related compounds or any nitrogen-bearing chemicals until it is proven conclusively by scientific means that these chemicals can be tolerated and in what concentrations. I am differing with the British Horological Institute in regard to the amount of ammonia that can be safely tolerated and I feel that my stance is quite valid considering the lack of quantitative scientific information that we have that directly relates to the interaction of ammoniated cleaning solutions and the brass components of clocks and watches. Guessing about the effects that ammonia can have is not appropriate when it concerns the preservation of our horological heritage!
When I clean clock components, I remove the dirt, deteriorated lubrication, and other deposits by washing them in an aliphatic hydrocarbon2 such as Stoddards Solvent or mineral spirits and using sharpened peg wood or soft bristle brushes. If a deposit is particularly stubborn, then I use a soft wire brush: a brass brush for brass and a steel brush for steel. One should never use a brass brush on steel as it will cause corrosion: the inverse is also true!
This process leaves a chemically clean surface without removing the “patina” on the parts. This solution will not etch the surface of the brass. Visually it looks like nothing was done; it does leave one feeling like the clock has not really been “cleaned” but in fact it is very clean. If I need to remove the surface oxidation or fingerprints (which is seldom), I use a very finely graded chalk dust (calcium carbonate) mixed into a paste with Stoddards Solvent, a brush, cotton swabs, and judicious pressure and care. I rinse the components thoroughly with a compatible rinse (Stoddard’s Solvent)!
Conclusion
Any commercial cleaning solution needs to be CHEMICALLY ANALYZED or the MSDS sheet examined prior to use to insure that ammonia, its related amines, or a nitrogen-bearing chemical is not present (even in minute amounts). You may request the MSDS sheet from the manufacturer of the solution or their distributor. They are required, by law, to supply this information because ammonia and many other chemicals that are used in various commercial recipes can be considered a health hazard. If you do not use commercial solutions, then do not mix up any solution that contains ammonia or amines or nitrogen-bearing chemicals in its recipe. If you do not understand the MSDS sheet or do not know what the chemicals are, contact a chemist or a metallurgist. Be cautious; do not assume anything!
An Additional Note
In addition to ammoniated cleaning solutions, one must AVOID THE USE OF POLISHING COMPOUNDS SUCH AS BRASSO** and NOXON** as well as GLASS CLEANING SOLUTIONS (such as WINDEX***) THAT CONTAIN AMMONIA OR ANY OF ITS RELATED COMPOUNDS. We have all seen dial rings that are cracked in hundreds of places (these cracks are not necessarily caused by the spinning operation that induces residual stresses but are most likely caused by the use of ammoniated polishing compounds and glass cleaning solutions).
Proposal
Because this is a universal problem throughout the world, I would like to propose a research project on safe cleaning solutions funded by the AWI, the BHI, the NAWCC, and any other horological organization (not connected with the cleaning solution industry) as well as Conservation Organizations and run and organized by an independent research laboratory that specializes in metallurgical failures due to chemical reactions. NOTE: as of 2003, this proposal has fallen on deaf ears…… no action has occurred!
Related Bibliography
(The following references can be used to further your understanding of SCC. These are just a few of the many that are available! I would encourage you to do further reading on this subject. SCC is not conjecture and is not a debatable subject; it is a fact.)
Symposium on Stress-Corrosion Cracking in Metals, Amer. Soc. Test Mater.-Amer. Inst. Min. (Metal.) Engrs., Philadelphia (1944) Corrosion, Sherir, L.,L.,: Newnes-Butterworth, 1976 The Corrosion Handbook, Uhlig, Herbert H.: John Wiley & Sons, New York Metals Engineering Design, Horger, Oscar J.: McGraw-Hill Book Co. Handbook of Ocean and Underwater Engineering, Myer, John (Editor): McGraw-Hill Book Co. Encyclopedia of Chemical Technology: Volume 7, 3rd Edition, pgs. 48-90, pg. 131: John Wiley & Sons, New York Metals Handbook, Ninth Edition, Volume 13, Pgs. 614-617, ASM International, Metal Park, OH Conservation of Clocks and Watches, Pgs. 27-28, Wills, P (Ed.), BHI 1995
Sources of Definitions (a) McGraw-Hill Encyclopedia of Science and Technology; 6th Edition, Volume 1, McGraw-Hill Book Co. (b) The Facts of File Dictionary of Chemistry, IBN 0-8160—2367-0 (c) Concise Encyclopedia Chemistry, Walter de Gruyter, Berlin, N7 1944
Source for chemicals:
For Stoddards Solvent, Mineral Spirits, Methanol, IsoPropanol: VWR Scientific 1-800-932-5000
For Mineral Spirits: Your local hardware store BUT beware that hardware store mineral spirits can contain a mixture of chemicals and thus is not a pure hydrocarbon.
Author’s Addendum: (not in published article) IMPORTANT NOTES:
1. Do not experiment with other cleaning solutions, you and I will probably not live long enough to see the results.
2. Just because the supplier says that it is okay does not mean that it is okay.
3. Since this article was written, I have amassed a collection of SCC examples that Ern has graciously agreed to include on his WEB site. I would encourage you to examine them carefully so that you can identify SCC.
4. Also, since this article was written and in order to test the susceptibility of clock movements to ammonia, I purposely exposed a 19th century Connecticut style clock to one teaspoon of household ammonia (you know……..the type that Mom uses around the house) for a period of 1 day. As a result, almost every brass component in this clock was cracked and in some cases the component was cracked in a multitude of places. The movement was completely ruined!
5. I also performed the same experiment with a 1989 Hermle clock movement using the same methodology and , although it took 5 days, the results were the same: it was riddled with SCC and the movement was ruined.
6. Rinsing should be done after washing. You must use a compatible rinse: remember like dissolves like. For my first two rinses, I use a 50/50 mixture of Methanol and IPA and the last rinse of just pure IPA. Make sure that you purchase IPA from a chemical house and not the local store because the chemical house brand, although more expensive, has a significantly smaller amount of moisture in it: i.e. it is 98% IPA and 2% water. If the water content is too high, the steel components will rust. I dry the components using warm air. Change the rinses often even though they look clean to the eye.
7. Your comments and questions are welcome.
Regards,
James Moss
Postscript:
Father & Son Precision Time was among many whose mainstay was an “off the shelf” concentrated ammoniated cleaning product (Daniel’s solution) sold by clockmaker supply firms. I’ve known Jim since 1986 when I brought him a damaged escape wheel from an E. Howard master clock. I respect his work, expertise and advice. While I’m “tinkering” with clock projects, Jim is doing research in his “laboratory”.
Jim Moss and his wife, Pam, visited with us a few years back. During Jim’s visit, he got the grand tour of my workshop. He had opportunity to “twist my arm” with respect to ammoniated cleaning solutions and stress corrosion cracking (SCC), but it’s not Jim’s nature to push folks. He shared briefly with me about the affects of ammonia on clock brass based on his research, but this was only after I questioned him. Eventually I visited his new shop (laboratory) in Littleton, Massachusetts where a new world of research opened before me and literally “opened my eyes” to the effects of ammoniated cleaning solutions on brass.
In March 2003, Jim shared his research with the membership of Maine Chapter 89, NAWCC, and though there will be stubborn curmudgeons who will never change, there were many converts in the chapter who will now seek the safest means to repair and restore timepieces and other collectibles made of brass.
I’m among the converts. This is enough from me. I admonish you to carefully read and re-read the following article with an open mind. It will change the way you work in your shop. Ern Grover, Father & Son Precision Time, Harrisonburg, VA.
To contact Ern by email, click here.
Though I encourage the conservative approach to movement cleaning, some customers want the brilliant look. I’ve developed an alternate and safe method for achieving these results without the harmful use of ammonia or water. Click HERE
|
|
Pictorial Documentation (click on any image for a larger view) |
|
|
Figure 1. At a magnification of 5.44X and within the range of a jeweler’s loupe, is showing a 12-tooth segment of a 60-tooth wheel. Close visual examination of the photograph will reveal a plethora of cracks all over this surface of the gear. This cracking has been determined by metallurgists to be Stress Corrosion Cracking (SCC). There are many different types of metallurgical failures (such as season cracking) and almost every type is easily identifiable. |
Figure 2. At a magnification of 23.7X, is a closer examination of the SCC located underneath the replaced tooth as shown in the rectangle of Figure 1. Note the light color at the top of the photograph (see Figure 2); it is the solder used to hold the replaced tooth in place. The SCC is clearly visible. |
|
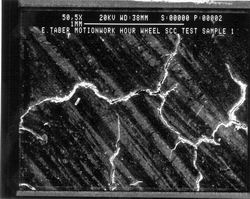
Figure 3. At a magnification of 50.5X, is yet a closer examination of the SCC located in the target area as shown in Figure 2. The light and dark stripes on the surface are the marks left by the file used to level the new tooth to the surface of the original gear. |
Figure 4. At 104X, is clearly showing intergranular cracking in the target area as shown in Figure 3. In general terms, intergranular cracking occurs when the bond between the grains of the metal is destroyed as a result of a corrodent chemical action and tensile stress. SCC is generally known for intergranular cracking and lesser known for transgranular cracking. Interesting to note is the fact that only “alloys” are subject to SCC and because brass is an alloy of copper and zinc, it is susceptible to SCC. |
|
Figure 5. At 205X, is the beginning to show the vertical cleavage of the SCC failure along the granular boundary between the grains of metal. |
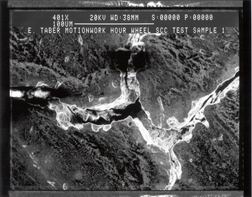
Figure 6. At 401K, presents an intergranular cavity, which clearly shows the SCC failure. |
|
Figure 7. At 803X, presents an incredible vertical cavity into which a corrodent chemical, such as ammonium hydroxide, can enter. These cracks extend through the full thickness of the hour wheel! The wheel is riddled with replacement teeth. The reason that the teeth were replaced at such a high rate is because SCC weakened the roots of the teeth and caused them to break off. Individual molecules cannot be seen with a Scanning Electron Microscope. They are too small (even at 803X!). This means that an incredible number of corrodent molecules could easily enter this crack! |
Author: James Moss, Horological Conservator For additional reading, please take a look at Jim’s article “Concerns Regarding the Conservation of Functional Horological Objects” as published with Stanford University. (Posted with permission James Moss, 1/12/2005)
|